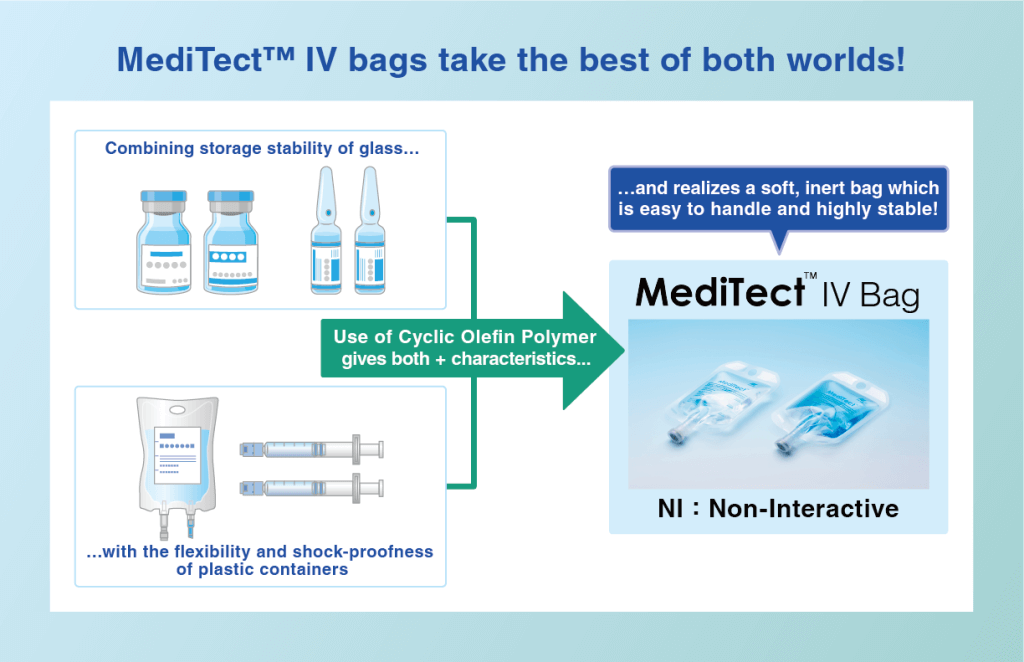
When changing the primary packaging of a product from glass to plastic, container-content interaction is the main concern, especially injectable drugs known to be unstable in plastic. MediTect™ series gives glass-like ultimate stability to more sensitive drug contents with more “flexible” solutions.
Our MediTect™ brand has been created specifically for sensitive liquid parenteral formulations. It offers a variety of material options and properties, making it ideal for premixed bags. We can also provide related accessory products, such as closures and overwraps.
See Shelf-Life Performance Results
Functionality
Containing proprietary cyclic olefin polymer technology, MediTect™ products combine an unparalleled level of chemical compatibility and sorption resistance with an ultra-low extractables profile. These properties provide product integrity and help maximize the shelf life for the most demanding applications.
- Ultra-low interaction
- Safe materials
- Alternative to rigid containers
- Multiple sterilization methods

Our Commitment to Quality
We have sold primary packaging for parenteral drugs to the US and Japan for over 30 years, and some products are DMF registered.
Our primary pharmaceutical packaging manufacturing site is ISO 15378 and ISO 13485 certified. Maintaining equivalent quality levels to our pharma customers and operating a one-machine-per-room philosophy, we deliver a new level of value and quality assurance in our products.